- Written by Super User
- Category: #3 (05) 2016
- Published: 18 April 2018
- Hits: 2084
Mokbel T. H.1, Ghanem A. A.1, Mady S. A.2, El-Emam D. S.1
1Mansoura Ophthalmic Center, Faculty of Medicine, Mansoura University, Mansoura, Egypt
2Ophthalmology Department, Faculty of Medicine, Benha University, Benha, Egypt
Purpose. To compare the biomechanical properties of the cornea and corneal sensitivity after small-incision lenticule extraction (SMILE group) with those after femtosecond laser-assisted laser in situ keratomileusis (femto-LASIK group).
Materials and methods. One hundred and fifty patients (80 eyes and 70 eyes) that received SMILE and femto-LASIK procedures, respectively, were enrolled prospectively in this study. Corneal biomechanical properties such as corneal hysteresis (CH), corneal resistance factor (CRF), P1 area, and P2 area were quantitatively assessed with the Ocular Response Analyzer. Corneal sensitivity was quantitatively assessed with the Cochet – Bonnet esthesiometry. All eyes assessed preoperatively, 1 week, and 1, 3, 6 and 12 months postoperatively.
Results. The decrease in CH, CRF, P1 area, and P2 area was statistically significant one week postoperatively compared with preoperatively in both groups (P < 0.05). However, these values in the SMILE group were significantly higher than those in the femtoLASIK group 3 months, 6 months, 12 months postoperatively (P < 0.05). Both SMILE and femto-LASIK eyes demonstrated impaired central corneal sensitivity and tear-film break up time immediately after both procedures. Central corneal sensitivity and tearfilm break up time values in the SMILE group were significantly higher than those in the femto-LASIK group 3 months, 6 months, 12 months postoperatively (P < 0.05). There were statistically significant correlations between the changes of postoperative central corneal ablation depth, and preoperative spherical equivalent at 12 months follow up in both groups (P < 0.05).
Conclusion. Both SMILE and femto-LASIK procedures can alter the biomechanical properties of the cornea. Changes in the corneal status were less after SMILE than after femto-LASIK. Both central corneal sensitivity and tear-film break up time impaired where the impairment less after the SMILE than after femto-LASIK.
Key words: cornea, biomechanical parameters, sensitivity, SMILE, femto-LASIK.
INTRODUCTION
The structural and reparative properties of the cornea are essential to its function as a resilient, barrier to intraocular injury. Because the cornea is also the major refractive surface of the eye, any mechanical or biological response to injury will also influence optical performance. Thus, the same mechanisms responsible for preserving ocular integrity can undermine the goals of achieving stable visual outcomes after keratorefractive surgery [1].
Great advances in the technique used in refractive surgeries to correct myopia have been made from LASIK procedure to that refractive lenticule extractions; small-incision lenticule extraction (SMILE), femtosecond laser-assisted LASIK (femto-LASIK).
The femtosecond laser, a relatively new technology in medicine, has rapidly become accepted as a safe and effective technology to create corneal flaps for laser in situ keratomileusis (LASIK) [2] by delivering precise laser pulses at a pre-determined depth in the cornea. Although early studies of femtosecond laser-assisted LASIK have shown good results and quick visual recovery, the creation of a corneal flap in a typical LASIK procedure has been shown to weaken the corneal biomechanical status [3].
Recently, this technology has been used in a new corneal refractive procedure, refractive lenticule extraction to correct myopia. SMILE is a new procedure of refractive lenticule extraction that developed totally without excimer laser support. SMILE characterized by performing lenticule extraction through a small incision [4] is expected to offer better biomechanical stability than procedures that involve flap creation, such as LASIK.
With the introduction of the VisuMax femtosecond laser in 2006 [5], keratorefractive surgery was revolutionized and femtosecond intrastromal keratomileusis was reinvented in the shape of refractive lenticule extraction [6].
The commercially available Ocular Response Analyzer (ORA), a non-contact dynamic bidirectional applanation device, has been used to assess the biomechanical status and parameters of the cornea, and in refractive corneal surgery to follow intraocular pressure (IOP) [7, 8]. ORA uses metered collimated air pulse to produce applanation of the cornea. There is an initial applanation phase, beyond concavity and rebound through a second applanation [9]. An infra-red electro-optical system is used to record inward and outward applanation events. Data from the air pulse stimulus and infrared detector is used to calculate biomechanical parameters such as corneal hysteresis, corneal resistance factor, Goldmann-correlated IOP, and corneal-compensated IOP [10, 11].
The cornea is one of the most densely innervated peripheral tissue in humans. Subsequent femtosecond or excimer lasers cut sub-basal and superficial stromal nerve bundles. It is believed that the decrease in the corneal sensation after keratorefractive surgery are closely linked to the surgical amputation of the corneal nerve fibers that is produced by the creation of the flap during refractive surgery, regardless of the flapcutting method is used [12, 13, 14]. Therefore, corneal sensation is decreased while the nerve regenerate.
PURPOSE
To assess the corneal biomechanical changes and corneal sensitivity after small-incision lenticule extraction procedure and compare the changes with femtosecond laserassisted LASIK.
MATERIALS AND METHODS
Study design
This was a prospective, non-randomized, and comparative clinical series. After explaining the details of the study, we obtained written informed consent from all patients before enrollment. The study was approved by Al Nour Femto Laser Center and, trust ethics committee and was carried out in accordance with the Declaration of Helsinki (1989) of the world medical association.
Patients
This prospective study included eyes with myopia and/or myopic astigmatism scheduled to have small-incision lenticule extraction (SMILE group) or femtosecond laser-assisted LASIK (femto-LASIK group).
Inclusion criteria included (1) Patients with age of 21 years or more, (2) Patients with a stable refractive error for at least six months; with manifest spherical equivalent of –1.00 to –10.00 diopters, manifest cylinder of –0.50 to –5.00 diopters, (3) Patients with a sufficient corneal thickness was greater than 500 um; minimum calculated residual corneal stromal bed thickness was greater than 300 um, (4) Intraocular pressure of 21 mm Hg or less, and (5) Patients discontinued soft contact lens wear at least two weeks before surgery.
Exclusion criteria included (1) Patients who had undergone intraocular surgery, (2) Patients with severe dry eye, progressive corneal degeneration, keratoconus, (3) Patients with lacrimal drainage disorders, and (4) Pregnancy or breast feeding, (5) Patients with diseases that affect the regenerative process of the cornea (diabetes mellitus, collagen related disease).
Patients underwent eye examination including slit lamp examination (Slit lamp BM 900, Haag-Streit, Bern, Switzerland), dilated fundus examination using a 90-diopter lens, and manifest and cycloplegic refraction and optimal visual acuity using a Snellen chart. Assessment of uncorrected distance visual acuity (UDVA) and corrected (CDVA) using an automatic refractometry (Nidek, Co.LTD, GAMAGORI, Japan), intraocular pressure (IOP) using a non-contact tonometer (CT-80, Japan). The preoperative central corneal thickness, keratometry, and anterior and posterior corneal elevation were measured using a Scheimpflug topography camera (Pentacam HR, Oculus Gmbh, Germany).
Corneal biomechanical parameter measurements
The Ocular Response Analyzer (ORA Richert Inc., Buffalo, NY, USA) [15] was used to measure corneal hysteresis (CH) and corneal resistance factor (CRF) values, using the dynamic bidirectional applanation device, preoperatively and at all postoperative visits. The instrument reveal two applanation pressure measurements (P1 and P2) done by applanating the cornea with a puff of air and recorded by an infrared signal. The main parameters analyzed from this device are CH, represents the ability of the cornea to absorb or dampen an applied force and can be calculated as the difference between P1 and P2, while CRF is a viscoelastic parameter expressed by the following equation: CRF = k1 x (P1 – 0.7 x P2) + k2, where k1 and k2 are constants. Three measurements with consistent signal quality were obtained, and the CH and CRF values were averaged for statistical analysis [7,16].
Corneal sensitivity assessment
Corneal sensation was measured with a Cochet – Bonnet esthesiometer (Luneau, Paris, France). This instrument consists of a nylon monofilament that is 60 mm in length and with diameter of 0.12 mm. The instrument was advanced perpendicular to the central surface of the cornea until contact between the instrument and the cornea was made. If the patient felt the filament, the response was defined positive. Corneal sensitivity was tested three times with each filament length, and the length of the filament was sequentially reduced from 60 mm in 5-mm steps. At least two positive responses among three attempts were considered a positive result at each filament length. The longest filament length that resulted in a positive result was considered the corneal threshold. All of the measurements were performed during slit lamp examination.
Tear-film Breakup Time
Tear-film stability was assessed based on Tear-film Breakup Time (TBUT). A fluorescein impregnated strip (Jingming, Tianjin, China) that had been wetted with nonpreservative saline solution was placed in the lower conjunctival sac, and the patient was asked to blink several times [17]. Using slit-lamp biomicroscopy with a cobalt blue filter, the time that elapsed before the first observation of tear film break up after a complete blink was recorded as the TBUT. The test was repeated three times, and the average of the three measurements was calculated. Corneal fluorescein staining was graded as described by De Paiva et al. [18].
Surgical technique
The VisuMax (Carl Zeiss Meditec AG, Jena, Germany) femtosecond laser system was used to realize surgical refractive corrections for patients in the SMILE and femtoLASIK groups with a repetition rate of 500 kHz. The femtosecond laser was visually centered on the entrance pupil and a small curved interface cone was applied. All procedures were performed under topical anesthesia (preservative-free benoxinate hydrochloride 0.4 % eye drops) in all cases.
In the SMILE technique, after sterile draping and insertion of the speculum, the patient’s eye is positioned under the VisuMax surgical microscope. Afterwards, the table moves to the laser treatment position under an illuminated and curved suction contact glass (so-called treatment pack). While the patient fixates an internal target light for centration, the cornea is partly applanated by moving the table upward towards the curved contact glass. The surgeon observes this motion through the operating microscope and controls the movement with a joystick. Once an appropriate centration “center of the pupil”, the surgeon initiates the automatic suction. The patient continues to observe the blinking target green light even when the suction is being applied. The VisuMax femtosecond laser produces ultra-short pulses of light, at a repetition rate of 500 kHz, that are focused at a precise depth in the corneal tissue.
A plasma state develops with optical breakdown, and a small gas bubble is formed from the vaporization of tissue. A series of bubbles are created in a spiral fashion with a typical spot distance of 3-5 µm resulting in cleaving of tissue planes.
Four subsequent femtosecond incisions are performed: the posterior surface of the refractive lenticule, the lenticule border, the anterior surface of the refractive lenticule, and side cut incision. After the suction has been released, the patient is moved towards the observation position under the microscope. A thin spatula is inserted through the side cut over the roof of the refractive lenticule dissecting this plane followed by the bottom of the lenticule. The lenticule of the intrastromal corneal tissue was dissected through the 2-3 mm tunnel side-cut opening incision (usually supero-temporal). The lenticule is subsequently grasped with modified serrated McPherson forceps (Ceuder, CmbH, Heidelberg, Germany; design Blum M.) and removed.
Laser cut energy was approximately from 130 to 160 nJ and spot spacing ranged from 2.5 to 4.5 μm. Then a 40° to 60° incision located at 12-O’clock position was created to allow the lenticule extraction. The intended thickness of the upper tissue arcade was 100 µm, and its intended lenticule diameter was 7.5 mm, which 1 mm larger than the diameter of the refractive lenticule (6.5 mm). The side cuts made for access to the lenticule were set 90° apart at a width of 4.5 mm. At the end of the procedure, any redundant portions of the cap need to be distributed evenly to the periphery using a dry microspear to avoid mud-crack microfolds in the cap, that achieved through the slit-lamp of the VisuMax.
In the femto-LASIK technique, after standard sterile draping and insertion of the speculum to keep the eye open, the patient’s eye was positioned under the VisuMax femtosecond laser surgical microscope. The patient fixated on an internal target light in the microscope for centration, and the cornea was applanated by moving the table upward toward the contact glass. The patient continued to observe the blinking target green light even when the suction was applied. Femtosecond laser pulses with a typical pulse energy of approximately 110 nJ were delivered with a pulse repetition rate of 500 kHz. The pulses were focused at a precise depth in the corneal tissue, and the laser pulses created micro-photodisruption or an expanding bubble of carbon dioxide gas and water that in turn cleaved the tissue and created a plane of separation. A track distance and spot distance were 3.0 μm during flap creation and 1.5 μm during flap side-cutting. The created flap diameter was 8.0 mm, and flap thickness was set to 100-110 μm. Side-cut angle and hinge angle were 90° and 50° respectively. The hinges were set in a superior orientation with a hinge length of 4.0 mm. The flaps were created by laser scanning in spirals from the periphery to the center of the pupil superior ring 4 mm.
After completion of the procedure, a spatula was inserted under the flap near the hinge and the flap was lifted. The corneal stroma tissue ablation was performed with a scanning-spot excimer laser (Allegretto, Wavelight laser Tecnologie AG, Ex 500 Excimer Laser, Germany) using a tissue-saving function with a repetition rate of 250 kHz and a pulse energy of 150 nJ. After completion of the procedure, a spatula was inserted under the flap near the hinge and the flap was lifted. Finally, the flap was repositioned and the interface flushed.
After surgery for both procedures, all patients wore bandage soft contact lenses (ACUVE OASYS, Inc. FL, USA) until the next day of the operation. Postoperative topical medication regimens were identical for each eye and consisted of the administration of an ophthalmic solution of Vigamox 0.5 % eye drops (moxifloxacin hydrochloride, Alcon Inc., USA) 4 times per day for 7 days, Pred Forte eye drops 1.0 % (prednisolone acetate, Allergan Co., Mayo, Ireland) 6 times per day with a taper over the course of two weeks, and a non-preservative tear supplement Optive eye drops (carboxymethylcellulose sodium eye drops, Allergan, Inc., Irvine, CA, USA) 4 times per day for two months.
Follow-up
The following parameters were evaluated in all of the patients before surgery and 1 week, 1 month, 3 months, 6 and 12 months after surgery: CH, CRF, P1 area, P2 area, IOP, UDVA, CDVA, TBUT, and central corneal sensation. Each visit also included a slit-lamp examination, tonometry, corneal topography, ocular response analyzer and esthesiometer.
Statistical analysis
All data were analyzed with the SPSS version 15 (SPSS Inc., Chicago, IL, USA). Parameters were summarized as mean and standard deviation (SD) of the percentage difference from baseline. Comparisons of continuous variables were examined by independent Student’s t-test or Mann – Whitney U-test as appropriate, and a chi-square test was used for statistical analysis of categorical variables at the baseline. Taking preoperative spherical equivalent (SE) at baseline as the selected covariate and different times for measurements as the repeated factor. The Pearson correlation coefficient (r) was used to evaluate the correlation between variables. In addition, we evaluated the mean differences between the SMILE group and the femto-LASIK group before surgery and at each postoperative time point for each variable. The Bonferroni-corrected posthoc test was conducted to adjust the observed significant level for multiple comparisons. A P value of 0.05 or less was considered statistically significant.
RESULTS
One hundred and fifty patients (80 eyes and 70 eyes), age ranged from 22 to 35 years, were enrolled in the present study according to the inclusion/exclusion criteria as described. There were 80 eyes undergone small-incision lenticule extraction (SMILE group), and 70 eyes undergone femtosecond laser-assisted LASIK (femto-LASIK group). The number of eyes evaluated at each examination point in both groups. More than 95 % of patients were followed up for 12 months summerizes in the table 1.
The demographic and clinical features in the studied groups are: in total, 80 eyes of 80 patients (35 males, 45 females) who received SMILE procedure were included, and 70 eyes of 70 patients (30 males, 40 females) who received femto-LASIK procedure were included. There were no statistically significant difference between the SMILE group and femto-LASIK group regarding age, gender, manifest spherical equivalent, manifest cylinder, logMAR UDVA, logMAR CDVA (P = 0.67, P = 0.43, P = 0.45, P = 0.34, P = 0.15, P = 0.32, respectively). The mean preoperative IOP, K reading, CCT, pupil diameter, optical zone, and ablation zone were no statistically significant difference between both groups (P = 0.27, P = 0.53, P = 0.52, P = 0.21, P = 0.34, and P = 0.57, respectively).
At postoperative 12 months, UDVA and CDVA was –0.08 ± 0.05 logMAR (Snellen equivalent, 20/16.1), –0.07 ± 0.06 logMAR (Snellen equivalent, 20/15.6) in SMILE group and –0.06 ± 0.09 logMAR (Snellen equivalent, 20/17), –0.07 ± 0.05 logMAR (Snellen equivalent, 20/16) in femto-LASIK group. There was no difference between the two groups in the postoperative UDVA (P = 0.11), CDVA (P = 0.25).
Preoperative and postoperative corneal biomechanical parameters in both groups: at most time-points, the postoperative CH, CRF, P1 area, and P2 area values decreased statistically significant in both groups over preoperative values (P < 0.05).
In the SMILE group, the CH, CRF, P1 area, and P2 area values were statistically significant lowest 1 week after surgery (11.2 ± 1.3 versus 7.8 ± 1.2, 10.7 ± 1.4 versus 7.3 ± 1.5, 2945.61 ± 532.5 versus 2231.43 ± 452.2, and 2125.52 ± 432.6 versus 1642.35 ± 435.2, respectively). However, there was an increase in CH, CRF, P1 area, and P2 area at 3 months, at which time the value was statistically significantly higher than the 1 week values (P < 0.24, P < 0.35, P < 0.45, and P < 0.15, respectively). Multiple comparisons showed statistically significant differences in measurements between preoperatively and all postoperative times (P < 0.05); there were no statistically significant differences in CH, CRF, P1 area, and P2 area measurements between 3 months and 6 months postoperatively (P = 0.23, P = 0.45, P = 0.37, and P = 0.41, respectively), or between 6 months and 12 months postoperatively (P = 0.43, P = 0.41, P = 0.38, and P = 0.46, respectively).
In the femto-LASIK group, the CH, CRF, P1 area, and P2 area values were statistically significant lowest 1 week after surgery (10.9 ± 1.5 versus 7.2 ± 1.4, 10.3 ± 1.2 versus 6.9 ± 1.2, 2854.32 ± 432.2 versus 2132.46 ± 432.1, and 2054.21 ± 324.5 versus 595.35 ± 532.6, respectively). However, there was an increase in CH, CRF, P1 area, and P2 area at 3 months, at which time the value was statistically significantly higher than the 1 week values (P < 0.37, P < 0.39, P < 0.42, and P < 0.19, respectively). Multiple comparisons showed statistically significant differences in measurements between preoperatively and all postoperative times (P < 0.05); there were no statistically significant differences in CH, CRF, P1 area, and P2 area measurements between 3 months and 6 months postoperatively (P = 0.29, P = 0.35, P = 0.38, and P = 0.47, respectively), or between 6 months and 12 months postoperatively (P = 0.41, P = 0.42, P = 0.35, and P = 0.36, respectively). The CH, CRF, P1 area, and P2 area values in the SMILE group were higher than those in the femto-LASIK group at each postoperative time-points.
Preoperative and postoperative tear-film breakup time (TBUT) and corneal sensitivity parameters in both groups: at most time-points, the postoperative values decreased statistically significantly in both groups over preoperative values (P < 0.05). In the SMILE group, the TBUT and corneal sensitivity values were statistically significant lowest 1 week after surgery (8.13 ± 3.52 versus 4.78 ± 4.32, and 62.34 ± 13.45 versus 37.35 ± 16.32, respectively). However, there was an increase in the TBUT and corneal sensitivity values at 3 months, at which time the value was statistically significantly higher than the 1-week values (P < 0.14, and P < 0.35, respectively). Multiple comparisons showed statistically significant differences in measurements between preoperatively and all postoperative times (P < 0.05); there were no statistically significant differences in TBUT and corneal sensitivity measurements between 3 months and 6 months postoperatively (P = 0.49, and P = 0.43, respectively), or between 6 months and 12 months postoperatively (P = 0.31, and P = 0.38, respectively). In the femto-LASIK group, the TBUT and corneal sensitivity values were statistically significant lowest 1 week after surgery (7.97 ± 4.35 versus 3.98 ± 4.21, and 58.32 ± 5.43 versus 31.25 ± 13.35, respectively). However, there was an increase in TBUT and corneal sensitivity values at 3 months, at which time the value was statistically significantly higher than the 1 week (P < 0.12, and P < 0.41, respectively). Multiple comparisons showed statistically significant differences in measurements between preoperatively and all postoperative times (P < 0.05); there were no statistically significant differences in TBUT and corneal sensitivity measurements between 3 months and 6 months postoperatively (P = 0.43, and P = 0.41, respectively), or between 6 months and 12 months postoperatively (P = 0.39, and P = 0.48, respectively). The TBUT and corneal sensitivity values in the SMILE group were higher than those in the femto-LASIK group at each postoperative time-points.
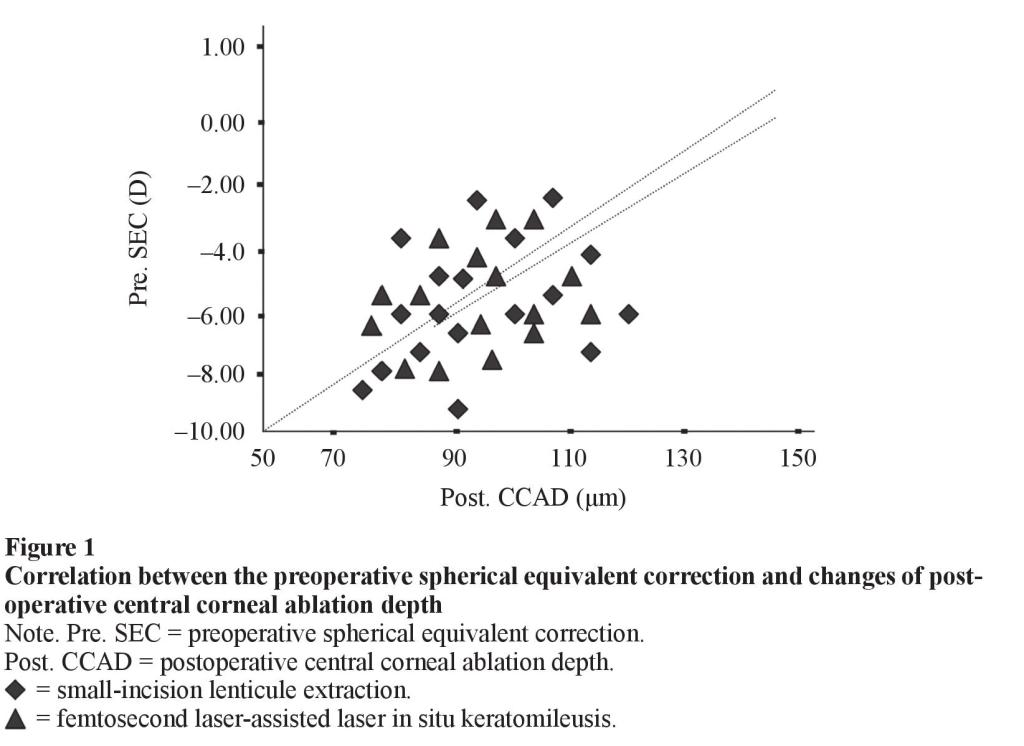
There was a statistically significant correlation between the preoperative spherical equivalent correction and changes of postoperative central corneal ablation depth parameter 12 months after SMILE procedure (Pearson correlation coefficient, r = 0.41, (P < 0.46)). A statistically significant correlation was also found between the amount of preoperative spherical equivalent correction and changes of postoperative, central corneal ablation depth parameter 6 months after femto-LASIK procedure (Pearson correlation coefficient, r = 0.35, P < 0.42) (Fig. 1).

DISCUSSION
A precursor to modern refractive lenticule extraction was first described in 1996 using a picosecond laser to generate an intrastromal lenticule that was removed manually after lifting the flap [19, 20], however, significant manual dissection was required leading to an irregular surface. It has been verified that flap creation and tissue removal can weaken the biomechanical properties of the cornea [21, 22]. Small-incision lenticule extraction, a procedure combining the flapless technique with the new concept of tissue subtraction, was generated to some extent by the need for biomechanical protection.
The purpose of this study is to assess the corneal biomechanical changes and corneal sensitivity after small-incision lenticule extraction (SMILE) procedure and compare the changes with femtosecond laser-assisted LASIK (femto-LASIK).
In the current study, we found a significant decrease in the biomechanical parameters after both SMILE and femto-LASIK one week postoperatively. After SMILE procedure CH, CRF, P1 area, and P2 area values were stable with no progressive deterioration after the 3 month follow-up. The same recovery was observed after femto-LASIK during the postoperative follow-up. In a study with a 12 months follow-up by Ryan et al., [23] CH after epithelial LASIK decreased significantly, with a slight recovery between 1 month and 6 months, then stabilized by 12 month postoperatively. Another 6-month follow-up study of the time course of corneal biomechanics after LASIK by Kamiya et al., [24] found that after the most significant changes occurred within 1 week postoperatively; the CH value was relatively stable.
CH is a dynamic measure of the viscous damping in corneal tissue, which represents the energy-absorption capability of the cornea. Both P1 and P2 areas showed a significant changes starting from 3 month postoperatively. This change was reflected on the significant changes of the CRF. The CRF is an indicator of the total corneal response, including the elastic resistance of the corneal tissue. Our findings indicate that SMILE, femto-LASIK may decrease not only the energy-absorption capability but also the elastic resistance of the corneal tissue in the early postoperative period, although no further changes occurred subsequently. We found that both techniques affected corneal biomechanical characteristics. This finding is in line with previous biomechanical results of keratorefractive surgery, such as LASIK and PRK [22, 26].
We observe that different responses between SMILE and femto-LASIK procedures are the result of differences in the wound-healing reaction. The CH value is thought to correlate with the viscous dampening inherent in the corneal tissues; the dampening is created by the viscosity of glycosamino-glycans, proteo-glycans, and the collagen matrix interaction [27]. Theoretically, the viscosity of the ground substance, which includes all the components of the extracellular matrix except collagen and elastic fibers, is the main factor that determines CH [16]. However, the CRF values changed less after SMILE than after femto-LASIK. This may attributed to the cornea is a highly complex anisotropic tissue with more extensive inter-lamellar branching in the periphery than in the center.
Biomechanically, the flapless lenticule extraction technique maximally protects the structural integrity of the cornea and causes less disruption of the peripheral collagen fibers than LASIK. Regarding the ORA methodology, the CRF is calculated by proprietary algorithms that place greater weight on P1; thus, this parameter is more reflective of the initial applanation event. The anterior cornea with integrated peripheral collagen fibers might provide stronger resistance than the cornea after flap creation [25]. This explained through the cohesive tensile strength testing of corneas directly showed that the stronger regions were located anteriorly and peripherally [26], and the anterior cornea with integrated peripheral collagen fibers might provide stronger resistance than the cornea after flap creation.
In the present study, the stronger correlation between the amount of preoperative spherical equivalent correction and changes of postoperative central corneal ablation depth, central corneal thickness indicates that the estimation of the thickness of the lenticule removed is more accurate with a femtosecond laser than an excimer laser. Estimating ablation depth with an excimer laser is difficult due to surgical swelling. In addition, we found significant correlation between the amount of preoperative spherical equivalent correction and changes of postoperative central corneal sensitivity. This may be attributed to the significant correlation between the amount of preoperative spherical equivalent correction and changes of postoperative corneal biomechanical properties that affects corneal sensation rather than the severing of the corneal nerves.
Corneal sensation reduction and dry eye are common after all types of corneal refractive surgeries [28]. In the current study, we observed time-dependent changes in the measurements of TBUT and central corneal sensation before and after SMILE procedure, and we compared these outcome measures with the same outcome measures in a group of patients who had undergone femto-LASIK treatment.
In the present study, we observed a significant 1 week postoperative reduction in TBUT, and central corneal sensitivity of patients in both SMILE and femto-LASIK groups compared with their preoperative sensitivity measurements. In addition, there was a trend toward increase of TBUT and corneal sensitivity postoperatively. The mean TBUT and central corneal sensation in the SMILE group was greater than that in the femto-LASIK group at all of the postoperative time-points, which indicated that deterioration of corneal sensation was greater after femto-LASIK than after SMILE. Our results were consistent with Wei et al. [29]. They found that the corneal sensitivity after SMILE surgery was better than that after femto-LASIK surgery at all postoperative visits (1 week, 1 month, and 3 months). Also, we found that at postoperative 3 months after SMILE and Femto-LASIK procedures, the TBUT and corneal sensitivity had recovered to nearly preoperative level.
Wilson et al., [30] supports the hypothesis that the most important factor in the pathophysiology of refractive surgery-induced dry eye and decreased corneal sensitivity is the transection of corneal nerves in the anterior third of the corneal stroma that occurs during the surgeries. In addition, the amputation of corneal nerves that occurs during refractive surgeries may subsequently result in the suppression of tear secretion from the lacrimal gland, mucin expression on the corneal epithelium, and frequent blinking because these homeostasis-maintaining behaviors are driven by a neuronal feedback loop that is mediated by corneal sensitivity [31, 32].
However, the most significant reason underlying less reduction corneal sensitivity after the SMILE procedure is less amputation of corneal nerves, which avoids the process of lifting a flap by extracting a lenticule from the stroma via a 3.0-mm incision, allowing less severing of corneal nerve fibers, whereas the femto-LASIK procedure requires the severing of all the superficial corneal nerves except the nerves that are located at the position of the hinge. Also, the epithelial-stromal-neural-lacrimal gland-immune cellular interactions are involved in the corneal response to injury after refractive surgery, the results of in vitro co-culture studies suggested that neurons and corneal epithelial cells support one another trophically through the mutual release of soluble factors [33].
REFERENCES
- Dupps WJ, Wilson SE. Biomechanics and wound healing in the cornea. Exp Eye Res 2006; 83:709–720.
- Stonecipher K, Ignacio TS, Stonecipher M. Advances in refractive surgery: microkeratome and femtosecond laser flap creation in relation to safety, efficacy, predictability, and biomechanical stability. Curr Opin Ophthalmol 2006;17:368–372.
- Shah S, Laiquzzaman M. Comparison of corneal biomechanics in pre and post-refractive surgery and keratoconic eyes by Ocular Response Analyzer. Cont Lens Anterior Eye 2009; 32:129–132.
- Shah R, Shah S, Sengupta S. Results of small incision lenticule extraction: all-in-one femtosecond laser refractive surgery. J Cataract Refract Surg 2011; 37:127–137.
- Blum M, Kunert K, Schröder M, Sekundo W. Femtosecond lenticule extraction for the correction of myopia: preliminary 6-month results. Graefes Arch Clin Exp Ophthalmol 2010; 248: 1019–1027.
- Vestergaard AH, Grønbech KT, Grauslund J, Ivarsen AR, Hjortdal JØ. Subbasal nerve morphology, corneal sensation, and tear film evaluation after refractive femtosecond laser lenticule extraction. Graefes Arch Clin Exp Ophthalmol 2013; 251: 2591–2600.
- Luce DA. Determining in vivo biomechanical properties of the cornea with an Ocular Response Analyzer. J Cataract Refract Surg 2005; 31:156–162.
- Chen S, Chen D, Wang J, Lu F, Wang Q, Qu J. Changes in Ocular Response Analyzer parameters after LASIK. J Refract Surg 2010; 26: 279–288.
- Pepose JS, Feigenbaum SK, Qazi MA, Sanderson JP, Roberts CJ. Changes in corneal biomechanics and intraocular pressure following LASIK using static, dynamic, and noncontact tonometry. Am J Ophthalmol 2007; 143: 39–47.
- Qazi MA, Sanderson JP, Mahmoud AM, Yoon EY, Roberts CJ, Pepose JS. Postoperative changes in intraocular pressure and corneal biomechanical metrics Laser in situ keratomileusis versus laser-assisted subepithelial keratectomy. J Cataract Refract Surg 2009; 35: 1774–1788.
- ElMallah MK, Asrani SG. New ways to measure intraocular pressure. Curr Opin Ophthalmol 2008; 19:122–126.
- Albietz JM, Lenton LM, McLennan SG. Chronic dry eye and regression after laser in situ keratomileusis for myopia. J Cataract Refract Surg 2004; 30: 675–684.
- Ambrosio RJ, Tervo T, Wilson SE. LASIK-associated dry eye and neurotrophic epitheliopathy: patho-physiology and strategies for prevention and treatment. J Refract Surg 2008; 24: 396–407.
- Wilson SE. Laser in situ keratomileusis-induced (presumed) neurotrophic epitheliopathy. Ophthalmology 2001; 108: 1082–1087.
- Agca A, Ozgurhan EB, Demirok A, Bozkurt E, Celik U, Ozkaya A, Cankaya I, Yilmaz OF. Comparison of corneal hysteresis and corneal resistance factor after small incision lenticule extraction and femtosecond laser-assisted LASIK: a prospective fellow eye study. Contact Lens and Anterior Eye 2014; 37: 77–80.
- Terai N, Raiskup F, Haustein M, Pillunat LE, Spoerl E. Identification of biomechanical properties of the cornea: the Ocular Response Analyzer. Curr Eye Res 2012; 37: 553–562.
- Chen F, Wang J, Chen W, Shen M, Xu S, Lu F. Upper punctal occlusion versus lower punctal occlusion in dry eye. Invest Ophthalmol Vis Sci 2010;51: 5571–5577.
- De Paiva CS, Pflugfelder SC. Corneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. Am J Ophthalmol 2004;137: 109–115.
- Krueger RR, Juhasz T, Gualano A, Marchi V. The picosecond laser for non-mechanical laser in situ keratomileusis. J Refract Surg 1998; 14: 467–469.
- Ito M, Quantock AJ, Malhan S, Schanzlin DJ, Krueger RR. Picosecond laser in situ keratomileusis with a 1053-nm Nd:YLF laser. J Refract Surg 1996; 12: 721–728.
- Qazi MA, Sanderson JP, Mahmoud AM, Yoon EY, Roberts CJ, Pepose JS. Postoperative changes in intraocular pressure and corneal biomechanical metrics; laser in situ keratomileusis versus laser-assisted subepithelial keratectomy. J Cataract Refract Surg 2009; 35: 1774–1788.
- Kamiya K, Shimizu K, Ohmoto F. Comparison of the changes in corneal biomechanical properties after photorefractive keratectomy and laser in situ keratomileusis. Cornea 2009; 28: 765–769.
- Ryan DS, Coe CD, Howard RS, Edwards JD, Bower KS. Corneal biomechanics following epi-LASIK. J Refract Surg 2011; 27: 458–464.
- Kamiya K, Shimizu K, Ohmoto F. Time course of corneal biomechanical parameters after laser in situ keratomileusis. Ophthalmic Res 2009; 42: 167–171.
- Wu D, Wang Y, Zhang L, Wei S, Tang X. Corneal biomechanical effects: Small-incision lenticule extraction versus femtosecond laser–assisted laser in situ keratomileusis. J Cataract Refract Surg 2014; 40: 954–962.
- Dawson DG, Grossniklaus HE, McCarey BE, Edelhauser HF. Biomechanical and wound healing characteristics of corneas after excimer laser keratorefractive surgery: is there a difference between advanced surface ablation and sub-Bowman’s keratomileusis?. J Refract Surg 2008; 24: S90–S96.
- Nishimura M, Yan W, Mukudai Y, Nakamura S, Nakamasu K, Kawata M, Kawamoto T, Noshiro M, Hamada T, Kato Y. Role of chondroitin sulfate-hyaluronan interactions in the viscoelastic properties of extracellular matrices and fluids. Biochim Biophys Acta 1998; 1380: 1–9.
- Mertzanis P, Abetz L, Rajagopalan K, Espindle D, Chalmers R, Snyder C, Caffery B, Edrington T, Simpson T, Nelson JD, Begley C. The relative burden of dry eye in patients’ lives: comparisons to a U.S. normative sample. Invest Ophthalmol Vis Sci 2005; 46: 46–50.
- Wei S, Wang Y. Comparison of corneal sensitivity between FS-LASIK and femtosecond lenticule extraction (ReLEx flex) or small-incision lenticule extraction (ReLEx smile) for myopic eyes. Graefes Arch Clin Exp Ophthalmol 2013; 251: 1645–1654.
- Wilson SE, Ambrosio R. Laser in situ keratomileusis-induced neurotrophic epitheliopathy. Am J Ophthalmol 2001;132: 405–406.
- Donnenfeld ED, Solomon K, Perry HD, Doshi SJ, Ehrenhaus M, Solomon R, Biser S. The effect of hinge position on corneal sensation and dry eye after LASIK. Ophthalmology 2003;110: 1023–1030.
- Konomi K, Chen LL, Tarko RS, Scally A, Schaumberg DA, Azar D, Dartt DA. Preoperative characteristics and a potential mechanism of chronic dry eye after LASIK. Invest Ophthalmol Vis Sci 2008; 49: 168–174.
- Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res 2003; 76: 521–542.
Received: 16 Feb. 2016
Published: December 2016